Complete blood count and its utility in fever investigations
Fever is the manifestation of many infectious diseases. However, infections can be trivial or be life-threatening, although clinical findings may be similar. Fever can also have other underlying causes, not treatable with anti-infective agents such as antibacterial, antiviral, antifungal, or antiparasitic medications.
Laboratory diagnostics is one of the cornerstones of healthcare, and test results form the basis for patient diagnosis. Hematology analysis constitutes a cost-efficient tool that provides useful information to clinical assessments. As different infections can have diverse effects on the blood cells and as other causes of febrile illness may have insignificant or no effects on the blood cells, a complete blood count (CBC) is often included, together with other general infection biomarkers such as C-reactive protein (CRP), in early fever investigations. This paper describes the clinical utility of CBC testing in fever investigations and how results, in combination with other elements of initial patient assessment, can provide early indications for possible diagnosis and support decision-making for further testing and treatment plan.
Introduction
Severe infections constitute one of the most common causes of intensive care unit admissions in tropical countries (1). Of travelers returning from such regions, as many as 2%–3% are present with fever, with malaria being the most common overall cause (2). Dengue and typhoid fever are also important causes of febrile illness in returning travelers (3). Even though fever can be caused by a trivial infection, it could also be a manifestation of a rapidly progressive and lethal infection. Short time to diagnosis for early initiation of treatment can therefore be necessary to save a patient’s life (2). Although patient examination, together with routine laboratory diagnostics, may provide clues to diagnosis in initial medical evaluation, there can be a considerable overlap in clinical manifestation and laboratory findings for many infections (4). On the other hand, while fever is typical in most malaria patients, as many as 40% will not report fever in early disease investigation (2), and although an elevated leukocyte count would suggest a bacterial infection, uncomplicated typhoid fever, brucellosis, and rickettsia infections are associated with a normal or low WBC count (3). The combination of a detailed review of the clinical course, physical examination, and laboratory data is therefore crucial in determining the likely cause. Travel history, review of pretravel vaccines, incubation period, and mode of exposure can also provide useful information for diagnosis (Table 1).
Together with CRP, a CBC with a leukocyte differential count is typically included in early patient assessment. For example, CRP level and platelet count can be useful in discriminating between dengue and malaria, with a low CRP being indicative of dengue, whereas a low platelet count, together with an elevated CRP level, is more indicative of malaria (4). While neutropenia is a common feature in many tropical infections, leptospirosis is typically associated with leukocytosis (1). Many parasite infections are associated with eosinophilia, however, protozoan infections such as malaria are typically not (2). Before initiating an extensive investigation of a parasite infection though, a careful review of medical history can be in place, as eosinophilia can also be associated with drug intake, including over-the-counter drugs (2).
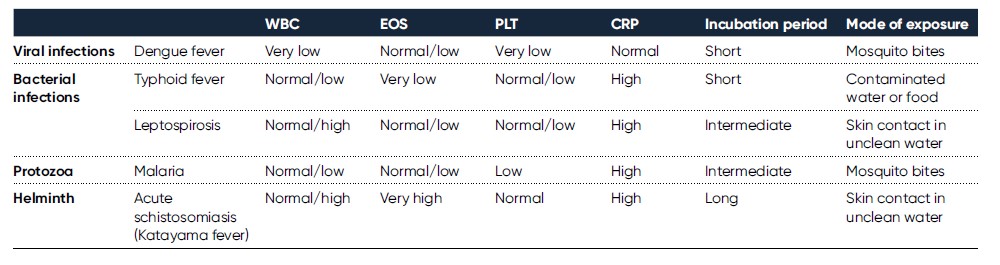
Table 1. Usual findings in assessment of patients suffering from febrile illness upon return from travel in tropical countries (2, 4, 5, 6, 7)
WBC EOS PLT CRP Incubation period Mode of exposure
Blood cells and their involvement in host defense
The hematopoietic system consists of organs and tissue involved in the production of blood components, mainly comprising plasma and blood cells such as erythrocytes (red blood cells), thrombocytes (platelets), and leukocytes (white blood cells). The blood cells are derived from pluripotent stem cells in the bone marrow, where they mature before being released into circulation. In healthy adults, about one trillion (1012) new blood cells are produced every day (Fig 1). The release of mature cells into circulation and their migration to various tissues are the result of organs communicating through the endocrine signaling system. A CBC therefore reflects the specific need for the different cell populations at a specific timepoint.
Myeloid cells
Myeloid stem cells give rise to cells such as red blood cells, platelets, monocytes, and granulocytes. Hemoglobin-rich erythrocytes are the most common blood cells (normal range ~ 4–6 × 1012/L). Their main function is to carry inhaled oxygen from the lungs to the tissues and organs of the body and remove carbon dioxide that is carried back to the lungs and exhaled. When the red blood cells are infected with a microorganism, they rupture, and hemoglobin is released. Red blood cells are also the target for the malaria parasite. However, recent studies of the ability of the red blood cells to bind pathogens indicate a more active role of these cells in the body’s immune response (8).
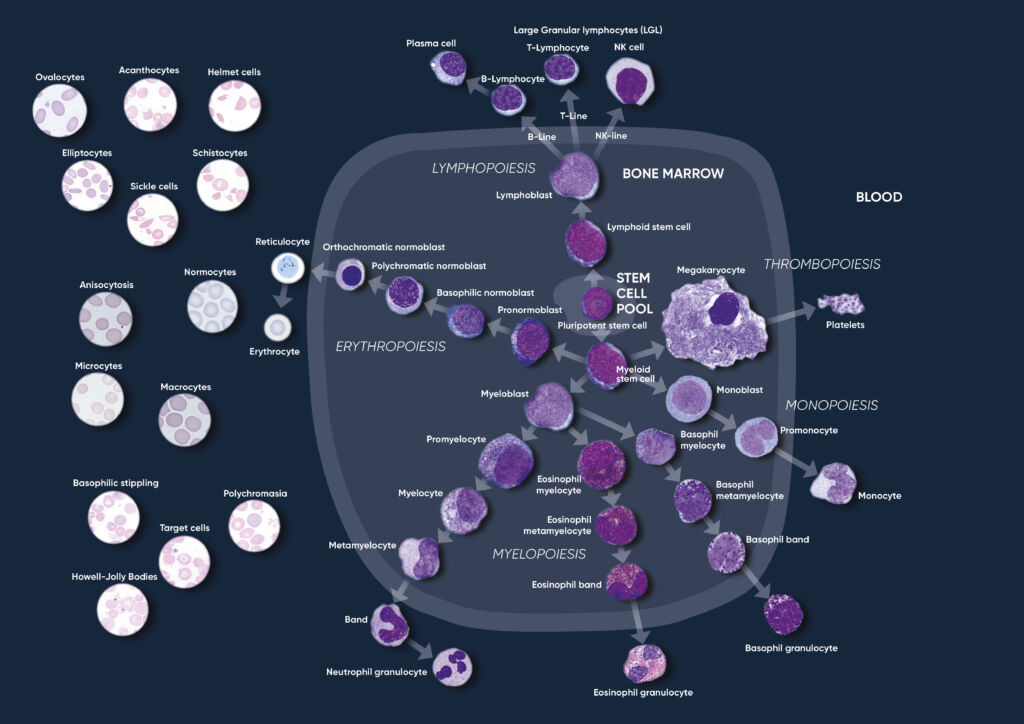
Fig 1. Overview of the hematopoietic blood cell production process (image created by Sigward Söderström and Helene Johansson, Örebro University Hospital, Sweden), which is growth factor-dependent and strictly regulated to maintain steady-state blood levels. Abnormally high or low levels are associated with different types of disease conditions.
The primary function of platelets (normal range ~ 130–400 × 109/L) is to prevent bleeding. They interact with each other and other cells, such as the white blood cells and vascular endothelial cells to search for sites of injury, where they become activated. Platelets also seem to be involved in the microbial host defense by detection and regulation of infection (9). When stimulated, their surface area is increased, and bioactive molecules are secreted. Platelets also seem to be target for the dengue virus, inducing platelet activation and apoptosis (10). A high number of monocytes (2%–8% normal) can indicate a chronic inflammatory disease or a bacterial infection. Their protective role includes phagocytosis, cytokine production, and antigen presentation. Monocytes have also been ascribed an active role in control of malaria infection by their ability to phagocytose infected red blood cells (11). In dengue, studies have shown that monocytes aggregate with activated and apoptotic platelets during the infection, which induces a specific cytokine response that may contribute to the pathogenesis of dengue (12).
Neutrophils, eosinophils, and basophils are three types of granulocytes that contain granules filled with proteins responsible for helping the immune system fight off infections. In normal blood, neutrophils constitute about 60% of the white blood cells (normal range ~ 3–10 × 109/L). As neutrophils help fight bacteria (and fungi), a high count can be an indication of an ongoing bacterial infection. Eosinophils (1%–4% normal) and basophils (0.5%–1% normal) are typically associated with allergies or parasite infections, and high counts are therefore associated with asthma, inflammatory reactions (especially those that cause allergic symptoms), and a present parasite infection.
Lymphoid cells
Lymphocytes originate from a lymphoid stem cell. In normal blood, about 30% of the white blood cells are lymphocytes such as B cells, T cells and natural killer (NK) cells. These cells are all necessary for the viral control, and a high count can therefore be indicative of an ongoing virus infection. Monitoring the lymphocyte count during a virus infection can also provide information about the body’s response to the infection.
CBC in diagnosis and monitoring of infectious diseases
As many infections affect the blood cells, initial fever investigations typically include a CBC. A CBC is also a cost-efficient analysis compared with more specific test, and therefore readily available in laboratories worldwide. Compared with more specific tests such as dengue PCR and NS1 antigen tests, malaria immunochromatographic test, or blood cultures that can take days to results (4), on-site CBC testing can provide results within a minute. The results help suggest a likely diagnosis, while waiting for confirmatory tests. As low numbers of white blood cells and platelets, together with a normal CRP, may help suggest dengue as a more likely diagnosis than malaria or typhoid fever, including such testing in early disease investigation supports the plan to wait for confirmatory tests before starting empiric antibiotic or antimalarial therapy (4). As many antibiotics may have some activity against plasmodia, taking such drugs can be associated with the risk of asymptomatic malaria or delaying the onset of malaria symptoms (3). As an elevated CRP value below 50 mg/L is not indicative of a bacterial or viral infection, a white blood cell differential count can be added to the CBC test. An elevated lymphocyte count would be an indication of a viral infection, whereas an elevated granulocyte count would suggest a bacterial infection (Fig 2). This information can also be useful for determination of the need for a blood culture. In bacterial infections, a blood culture is often requested to determine type of microorganism to allow selection of suitable antibiotic, ultimately preventing the spread of antimicrobial resistance. Considering classic fever as a temporary elevated body temperature, the cause can be other than an infection, but rather due to heat exhaustion, certain chronic inflammatory conditions such as rheumatoid arthritis, anemia, malignant tumors, medications such as antibiotics, or drugs used to treat high blood pressure or seizures. Fever is also a regular side effect of immunization. Immunization, heat exhaustion, and medication may have little or no impact on the CBC results. Tumors, anemia, and chronic diseases can change the CBC results, but changes are often specific for each nosology.
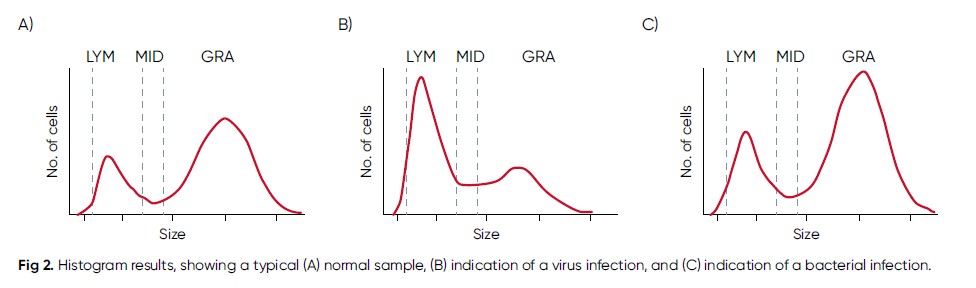
Fig 2. Histogram results, showing a typical (A) normal sample, (B) indication of a virus infection, and (C) indication of a bacterial infection.
In COVID-19 vaccination, it is important for general practitioners to differentiate between post-immunization fever and onset of COVID-19 virus infection. It can be hard to imagine that, for example, fevers caused by meningitis immunization and meningitis could coincide. However, with the current pandemic and mass vaccinations against COVID-19, it is a common case. Fever (temperature ≥ 38°C) was reported after the second COVID-19 vaccine dose in 16% of younger vaccine recipients and in 11% of older recipients, however, the systemic events (including fever and chills) observed within the first 1 to 2 days after vaccination were resolved shortly thereafter (13). If the temperature does not normalize in 3 to 4 days, it would make sense to perform a CBC test, followed by a COVID-19 specific test if found necessary, as this could be the start of a virus or a bacterial infection. Additionally, long-term inexplicable fever is an alarming signal for physicians to start the first screening of malignant tumors or chronic systemic disease, and a CBC is a part of this process.
Dengue diagnostic testing – a case study
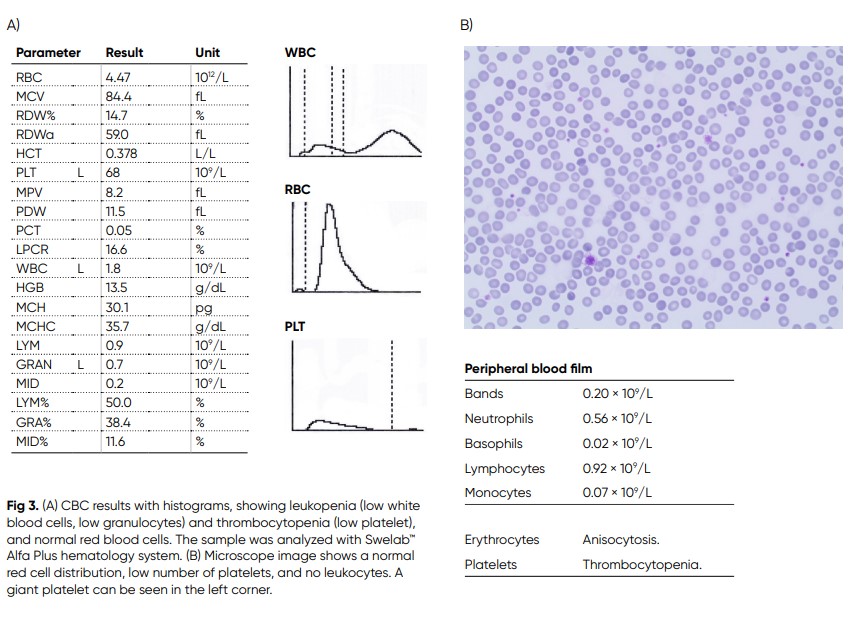
A Swedish male in his late twenties had been travelling in Thailand and Malaysia for five months, during which he got several mosquito bites (14). After returning to Sweden, due to emergence of a high fever with rigors, malaise, and myalgia, he was transported to the hospital. He had a fever of 39.0°C, and an erythematous rash on his back and shoulders was observed. Laboratory screening revealed neutropenia and thrombocytopenia (Fig 3). Occasional variant lymphocytes were seen in the blood film. CRP, lactate dehydrogenase (LDH), aspartate aminotransferase (ASAT), and activated partial thromboplastin time (APTT) were all slightly elevated. Microscopy was negative for malaria. Dengue fever was suspected, and later confirmed by the presence of IgM antibodies against the virus antigen. The patient was given intravenous fluid support and was observed for some days before discharged upon recovery of platelets to 68 × 109/L and white blood cells to 1.8 × 109/L. No spontaneous bleeding or severe plasma leakage, which are feared complications of Dengue fever, were developed.
Malaria diagnostic testing – a case study
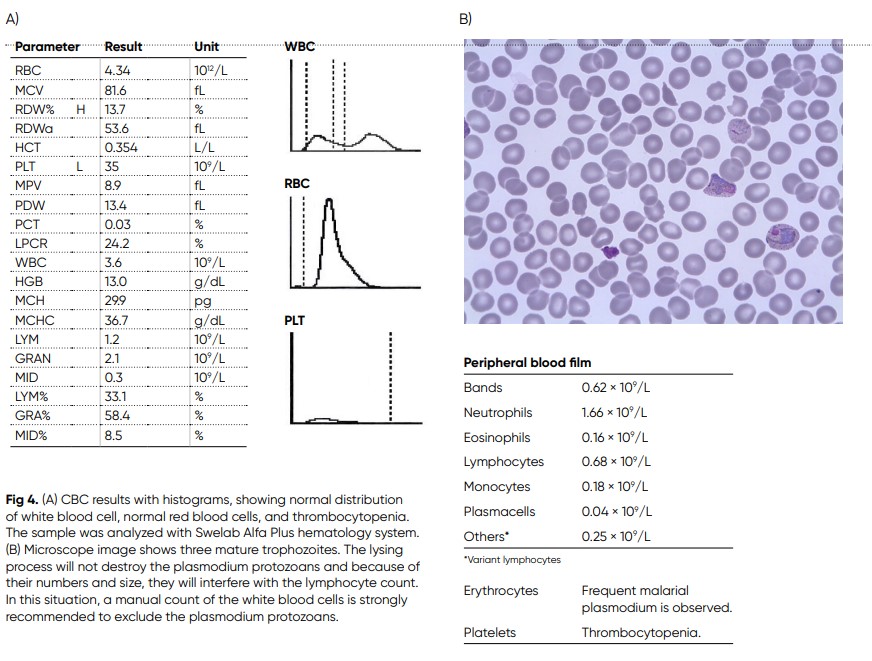
A 50-year-old man arrived at hospital with pain in his muscles and joints. Fatigue and coughing, and with fever that went up and down during nights (14). He had been travelling a lot in his work, and latest in central India with no recommendation for
malaria prophylaxis. Laboratory screening shows PLT 35 × 109/L, alanine aminotransferase (ALAT) 2.8 μkat/L, aspartate aminotransferase (ASAT) 3.0 μkat/L, bilirubin 31 μmol/L, and CRP 98 mg/L. The blood film shows trophozoites of Plasmodium vivax at all stages (Fig 4). The patient was treated by initially given artesunate (iv), and when patient got better, the treatment was changed to Riamet® and after a couple of days, also primaquine was given.
COVID-19 diagnostic testing
For practical and efficient screening of large populations for COVID-19 infection, initial reports from China recommended to include white blood cell count and CRP in laboratory examinations for early monitoring of infection (15, 16).
“Many factors contributed to developing our clinical algorithm in Wuhan during the early outbreak period”, Zhang and colleagues at the Department of Emergency Medicine,
Union Hospital, Wuhan, China explain in an article in The Lancet (16). “During this time, the influx of patients to fever clinics substantially outweighed the number of physicians.
Inpatient care was unsafe due to potential cross-infection and supplementary resources were not ready. Applying and waiting for results of a SARS-CoV-2 test was time consuming just after the outbreak and did not aid clinical decision-making.”
Learnings from China adopted in Honduras
Honduras is a country that has been hit hard with COVID-19 infections. Temporary tirage and testing centers are established throughout the country to cope with the increasing number of cases. Dr. Fernando Bonilla, Clinical Microbiologist at Permanent Contingency Commission, Triage Center Honduras uses the Swelab Alfa Plus hematology system in patient screening (Fig 5). “A blood count is a reliable, low-cost, and immediate reading test that helps us in the screening of people suspected of being infected by SARS-COV-2, and it is one of the first filters we use in decision-making”, says Dr. Bonilla. “Depending on the results of this and other laboratory exams, for example CRP, we use a confirmatory test for the final diagnosis of the patient. Thus, we have managed to contribute to an efficient use of the resources that are available in the temporary COVID testing centers in Honduras. Once we confirm a person positive for SARS-COV-2, we carry out a routine follow-up that usually includes up to three blood counts during the patient’s convalescence. By interpreting the results, we can see how the patient’s immune system is dealing with the viral infection and prepare for different scenarios, from cases that can be discharged soon to cases that may require intensive care. One of the most relevant parameters in SARS-COV-2 cases is the lymphocyte count, as these cells are the subgroup of white blood cells responsible for the management of infections caused by viral agents. In confirmed cases, we want to see an out-of-range count on the upper limit, which is indicative of an immune response in-line with the infection. A sudden drop in this population triggers an alarm that the patient could be entering into an acute phase of the disease.
Fig 5. Dr. Bonilla uses a Swelab Alfa Plus analyzer equipped with space-saving automation wheels to cope with high workloads, and with the MPA inlet that reports a full CBC from a finger-stick sample in about one minute.
Another important parameter in the study and management of positive cases is the platelet count. We have noticed that, in many serious cases, there is a clearly lower count that is outside of normal ranges. With Swelab Alfa Plus, we have a highly reliable instrument, easy to use, with an autoloader for the automated handling of a significant number of samples, and with the capillary blood functionality for urgent cases that require an immediate count and where a tube of venous blood sample is not necessarily available. In such cases, we can use a drop of blood from the patient’s finger to obtain a hemogram with 22 parameters in one minute.”
Conclusion
Many infections are associated with high mortality. Early suggestion for a likely diagnosis can therefore save lives. However, many infections share the same symptoms and clinical findings. The combination of a detailed patient anamnesis, physical examination, and laboratory data is therefore a prerequisite for identification of the likely cause.
A pandemic such as the COVID-19 outbreak poses additional challenges to disease investigations. A large number of patients needs to be screened to distinguish virus infected patients from healthy individuals. The fact that vaccination and actual infection both can cause febrile illness adds to the complexity. Techniques widely used in detection of infectious agents are PCR, serology testing, and feces and blood cultures. However, such methods can be both costly and time consuming. Additionally, sample collection can be difficult to perform and inconvenient for the patient. Together with general infection biomarkers such as CRP, a CBC, reporting also a white blood cell differential count, can be both a rapid and cost-efficient tool for early identification of infected patients. A CBC can also be useful in monitoring of disease progression and treatment efficacy.
Swelab Alfa Plus hematology system is intended for on-site patient screening. Its compact design makes the analyzer suitable for installation in confined spaces. Equipped with the micro-pipette adapter (MPA) inlet for analysis of capillary samples (20 μL), the analyzer reports 22 parameters from a fingerstick sample in about one minute. Capillary blood is often simpler to collect than a venous blood sample, and fingerstick sampling can be easier on the patient. The small volume also contributes to operator safety when handling infectious samples. Swelab Alfa Plus hematology testing, in combination with other elements of initial disease investigations, can provide a rapid and cost-efficient solution, supporting early decisions on further testing and treatment plan.
References
1. Tewari, H. and Nangia, V. Severe Tropical Infections. ICU Protocols pp 403–410. In: Chawla R., Todi S. (eds) ICU Protocols. Springer, India (2012).
2. Wilson, M.E. and Schwartz, E. Fever. Travel Medicine 513–521 (2008).
3. Gautret et al. Fever in Returned Travelers. Travel Medicine 495–504 (2019).
4. Cooper et al. Laboratory Features of Common Causes of Fever in Returned Travelers. Journal of Travel Medicine 21, 235–239 (2014).
5. Gherardin, A. and Sisson, J. Assessing fever in the returned traveller. Aust Prescr 3510–3514 (2012).
6. de Sainte Marie et al. Leptospirosis presenting as honeymoon fever. International Journal of Infectious Diseases 34 102–104 (2015).
7. Steiner et al. Acute Schistosomiasis in European Students Returning From Fieldwork at Lake Tanganyika, Tanzania. Journal of Travel Medicine 20, 380–383 (2013).
8. Anderson et al. The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity. J Immunol 201, 1343–1351 (2018).
9. Assinger, A. Platelets and Infection – An Emerging Role of Platelets in Viral Infection. Front Immunol. 5, 649 (2014).
10. Rondina, M.T. and Weyrich, A.S. Dengue virus pirates human platelets. Blood 126, 286–287 (2015).
11. Ortega-Pajares, A. and Rogerson, S.J. The Rough Guide to Monocytes in Malaria Infection. Front Immunol 9, 2888 (2018).
12. Hottz et al. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol 193, 1864–1872 (2014).
13. Polack et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. NEJM 383, 2603–2615 (2020).
14. Case Book: Swelab Alfa Plus. Boule Diagnostics 20813, Edition 3 (2019).
15. Lu, L. Interpretation of 7th edition of COVID-19 diagnostic and treatment guidelines. Lifotronic webinar: Diagnosis Guidelines for COVID-19, March 19, 2020.
16. Zhang et al. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. The lancet 8, Pe11-e12 (2020).
Disclaimer
Swelab Alfa Plus from Boule Diagnostics is an automated hematology analyzer for in vitro diagnostic use under laboratory conditions. Boule products do not make diagnoses on patients. Boule intends its diagnostic products (systems, software, and hardware) to be used to collect data reflecting the patient’s hematological status. This data, in conjunction
with other diagnostic information and the evaluation of the patient’s condition, can be used by a trained clinician to establish a patient’s diagnosis and to define clinical treatment.
The content of this paper was reviewed from a clinical perspective by Andrey Shanchev, MD, Boule Medical LLC, Russia. Acknowledgement We thank Kristina Nilsson, Reg. Biomedical Scientist, and Sigward Söderström, Reg. Biomedical Scientist, for collecting,
editing, and photographing material for the case studies. All material for these investigations had been taken from daily routine blood samples analyzed in the Hematology Laboratory, at the Department of Clinical Chemistry, Örebro
University Hospital, Sweden. We also thank Maria Åström, Hematologist, MD, for clinical evaluation and Maria Sjöberg, nurse at the infection clinic, for assisting with patient data
for the Section Parasitic infections. Cases are reviewed and diagnoses are adapted to the current WHO-classification by Helene Johansson, Reg. Biomedical Scientist, Sahlgrenska
University Hospital, Göteborg. We thank Fernando Bonilla, Clinical Microbiologist at
Permanent Contingency Commission, Triage Center Honduras for kindly sharing the clinical utility of CBC testing in screening of people suspected to be infected with SARS-COV-2 virus.
PDF download
White paper: White paper CBC in infectious diseases_WP_39211-2