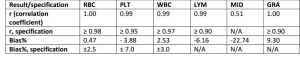
A comparison of performance of Medonic M32 with reference instrument for complete blood count (CBC) analysis.
Medonic M32 automated hematology analyzer is routinely used in laboratory diagnostics for determination of patients’ blood status. This work demonstrates the performance of Medonic M32 3-part hematology analyzer in comparison with a more technically advanced 5-part reference analyzer in complete blood count (CBC) analyses of patient samples taken from the normal routine screening. The results show that the analyzers are in good agreement, indicating the suitability for use of Medonic M32 in general health screenings.
Introduction
A CBC is highly useful in general screenings as a tool to aid in diagnosis and monitoring of disease conditions. Automated instruments for this type of analyses were developed as early as in the 1950s. The Medonic systems were introduced by Ingemar Berndtsson and Bram Bottema, founders of Medonic AB in 1982 (now part of Boule Diagnostics) and both with a long history and experience in hematology, clinical chemistry, and blood-banking engineering.
Before, blood cell counts were performed manually by microscopy. Although manual examination of blood smears is still used as a control method for verification of results from abnormal samples, the automated hematology analyzers have largely replaced the manual method for determination of hematology parameters in the routine use.
The Medonic M32 system is an automated hematology analyzer for in vitro diagnostic use under laboratory conditions (Figure 1). The analyzer is intended for determination of hemoglobin (HGB) concentration, for counting of red blood cells (RBC) and platelets (PLT) as well as for counting and differentiation of white blood cells (WBC) into three subpopulations, namely lymphocytes (LYM), mid-sized white cells (MID, mainly monocytes), and granulocytes (GRAN, mainly neutrophils, eosinophils and basophils). The measurement principles of the Medonic M32 are based on impedance for cell counts and spectrophotometry for HGB.
Although such a 3-part hematology analyzer provides enough information for the smaller local hospital laboratory, trends show an increased interest in 5-part instruments, typically used in larger central hospital and hematology laboratories, also for use in small physician office laboratories (POL).
While a 5-part analyzer offers improved WBC assessment, differentiating them into neutrophils (NEU), lymphocytes (LYM), monocytes (MONO), eosinophils (EOS), and basophils (BASO), a 3-part instrument can offer great cost benefits to general screenings of patients’ blood status (1).
The objective of this study was to evaluate the performance of Medonic M32 3-part hematology analyzer against a 5-part reference instrument.