Hematology testing in blood banking and transfusion applications
A blood transfusion can in many cases be the only therapy available to treat an acute or chronic health condition. However, such an intervention can be associated with risks, for example, of adverse reactions in recipient as a response to the transfused cells or infections transferred to the recipient. Blood banks and transfusion centers therefore undertake certain measures to ensure transfusion safety, and hematology testing constitutes an important part of the pre- and post-transfusion assessments as well as in quality control of the blood products. In this article, a brief background to hematology testing at blood banks and transfusion centers is given and the use of Medonic™ M32 automated hematology analyzer in donor screening is demonstrated.
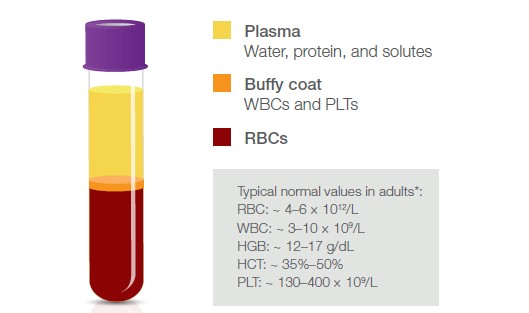
Fig 1. Blood fractions and their constituents. *It is good practice that each laboratory establishes its own reference values.
Introduction
A blood transfusion provides the patient with required blood components, for example, in the event of an acute blood loss (1) (Fig 1). Red blood cells (RBCs) can be given when hemoglobin (HGB) is low, for example, due to a disease, a bleeding caused by an injury or during surgery, or in case of iron-deficiency anemia. Platelets (PLTs) can be given to treat patients that are either low in PLTs or show poor PLT function. Whole blood is also sometimes given, for example, in case of a massive bleeding, in exchange transfusions, or when people donate blood to themselves (autologous blood transfusion). Whole blood transfusion from a donor to a recipient (allogenic blood transfusion) is often depleted from white blood cells (WBCs), that is, the leukocytes, when they are not considered to provide any benefit but rather constitute a risk of transferring bacteria and viruses to the recipient. With a panel of regular voluntary blood donors, which are screened for infectious agents, and with good manufacturing practices for the blood product, however, the risk of bloodborne infections can be minimized. Granulocytes (GRA) are, for example, sometimes given to patients to help fight life-threatening infections.
Ensuring transfusion safety
For blood transfusion safety, it is recommended that blood banks have in place an effective quality system that includes standardized procedures and good manufacturing practices, and to undertake specific measures to ensure correct labeling, storage, and transportation of the blood product (1). To further minimize the risks associated with blood transfusion, single-unit transfusions have been suggested to certain patient groups to prevent repeated exposure to blood products (2). Additionally, single-donor blood products have been suggested for transfusions, rather than pooled blood products from multiple donors, to reduce the risk of transfusion-transferable infections (3). Hematology testing is commonly used in quality-monitoring of the blood products. For example, PLT concentrates are typically assessed for residual leucocytes, and RBC products are additionally assessed for HGB and hematocrit (HCT) values (Table 1). As the WBC linearity range of many hematology analyzers exceeds the maximum WBC count allowed for leukocyte-depleted blood products, flow cytometry is commonly used as a supplementary method for such products. Minimum standards for blood establishments and hospital blood banks that are required to comply with European Union (EU) Directive 2005/62/EC are given in the Guide to the preparation, use and quality assurance of blood components (1).
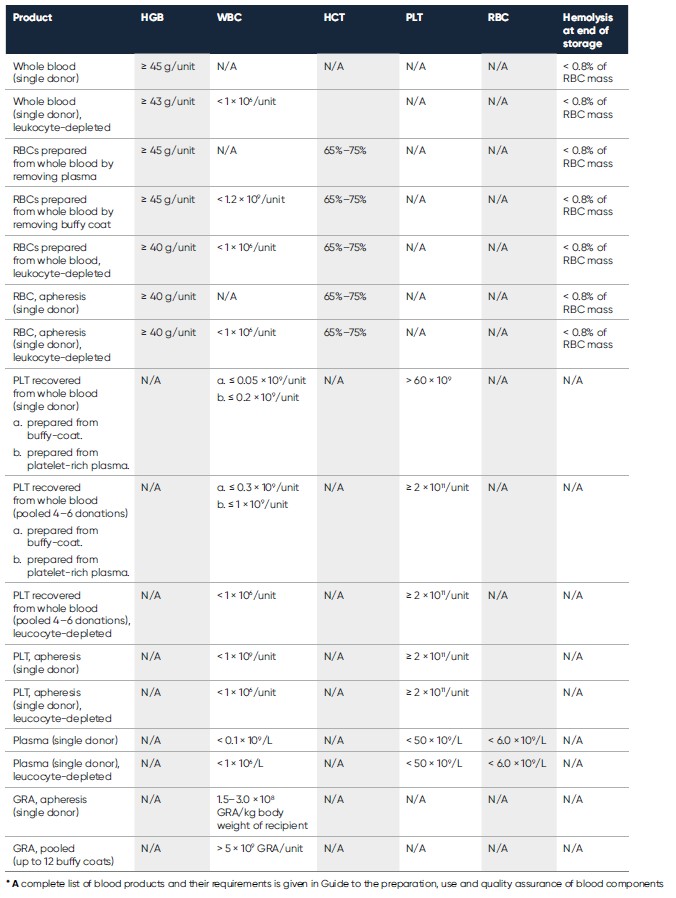
Table 1. Requirements for hematology parameters of some blood products intended for transfusion to adult recipients*
Pre-donation testing
Before each donation, a donor assessment is typically conducted, and only individuals of good health are selected (4). Donor-induced iron deficiency (DIID) is of particular concern, and it is recommended that RBC donations from individuals with pre-donation HGB below a certain threshold are deferred. In addition to the HGB value, other parameters that can aid in identification of DIID are the RBC indices, such as RBC size (mean corpuscular volume, MCV) and RBC distribution width (RDW) as well as HGB amount per RBC (mean corpuscular HGB, MCH = HGB/RBC) and concentration of HGB relative to RBC size (mean corpuscular HGB concentration, MCHC = HGB/HCT) (4, 5).
If accepted, the donor is also assessed for blood type to allow for determination of compatibility with the recipient, as well as for possible infectious agents such as HIV, hepatitis B, hepatitis C, and syphilis (1).
Pre-transfusion testing
As transfusion is associated with the risks of adverse reactions and blood-transmittable infections, it is recommended to carefully assess the patient for its actual need of a blood transfusion, as many conditions could also be managed by other means (6). Such an assessment can, for example, include testing of hematology parameters such as HGB and HCT to determine the cause of a suspected anemia and if the anemia can be treated with an oral iron course (Fig 2).
If a transfusion is prescribed, blood grouping and compatibility tests are normally conducted to ensure that transfused cells (e.g., RBCs) are compatible with the recipient’s antibodies and to avoid stimulating the production of new antibodies against the transfused cells (e.g., anti-RhD).
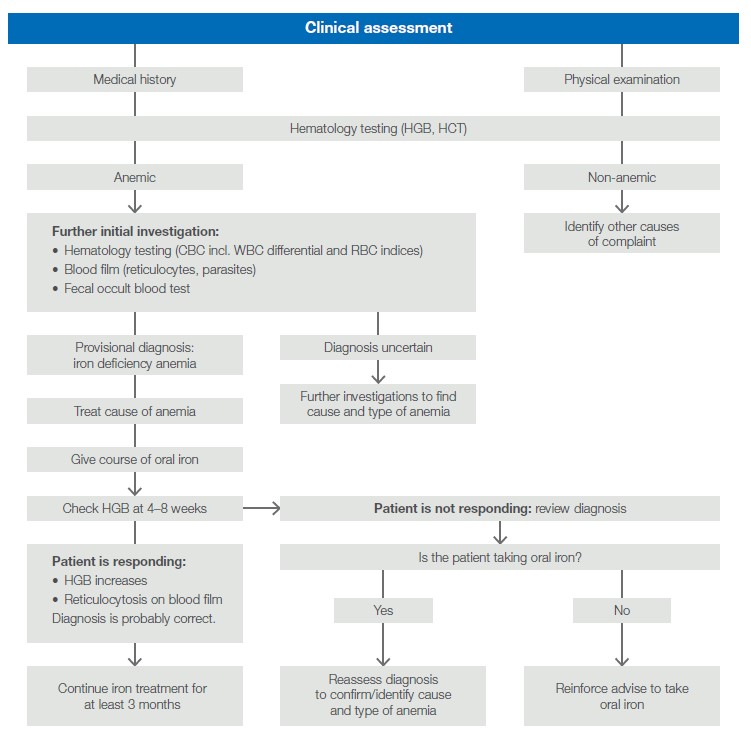
Fig 2. Clinical assessment to determine the cause of anemia. Adapted from Handbook: The Clinical Use of Blood (6).
Automated hematology testing in blood bank applications
The Medonic M32 system is an automated hematology analyzer intended for determination of HGB concentration and for WBC, RBC, and PLT counts. Additionally, the analyzer reports a range of other parameters such as the number and WBC percentage of lymphocytes, mid-sized white cells, and granulocytic white cells, HGB content of the RBCs, as well as volumes and distribution widths of the RBCs and PLTs.
The Medonic M32 measurement principles are robust and well-proven. Blood cell counts are based on impedance, and HGB is determined by photometry. The analyzer offers a wide choice of sampling methods: open tube (OT), pre-dilute, cap-piercing, autoloader, and micro-pipette adapter (MPA) sampling (Table 2).
Complete blood count from one drop of blood
The MPA method allows hematology testing from a capillary sample taken directly from the patient (Fig 3). Following general recommendations, the results obtained with capillary samples using the MPA inlet are comparable with those obtained with venous blood samples using the OT inlet (Table 3).
The MPA method reports a complete blood count, including a 3-part differential of the WBCs, from a simple finger-stick sample. This functionality makes Medonic M32 well-suited for use in donor and recipient assessments.
Donor acceptance study, comparing the OT and MPA sampling methods
In a study performed at BloodWorks Northwest Donor Center, Seattle, WA, USA, the MPA and OT sampling methods of Medonic M32 were compared in assessments of adult donors. Both female and male donors were included in the study that was conducted over the period from 8 to 18 August 2016.
Sample analyses show no male donors outside of limits with either the OT or MPA method. For female donors, three samples were observed outside of limits with the OT method, of which two were observed outside of limit also with the MPA method. The third sample that was shown to be outside of limits with the OT method was observed close to limits with the MPA method. The results indicate good agreement between the MPA and OT sampling methods (Fig 4). It shall be noted that the operator’s skill plays an important role in analysis of capillary blood.
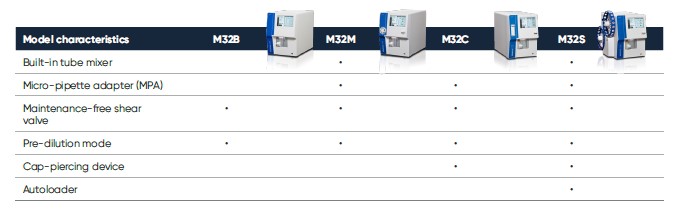
Table 2. Medonic M32, trusted for its high reliability and ease-of-use, is available in multiple models to fit your needs.
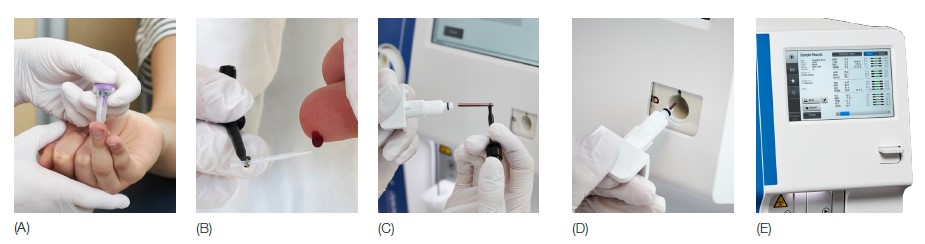
Fig 3. Medonic M32 automated hematology system equipped with the micropipette-adapter (MPA) inlet for capillary samples is designed for blood testing in a near-patient clinical setting. (A) Make a deep and firm skin puncture. (B) Collect 20 μL blood into the K2EDTA-coated micropipettes. (C) Slide the tube into the adapter. (D) Insert the adapter into the analyzer. (E) CBC results are viewable on the display in about one minute.
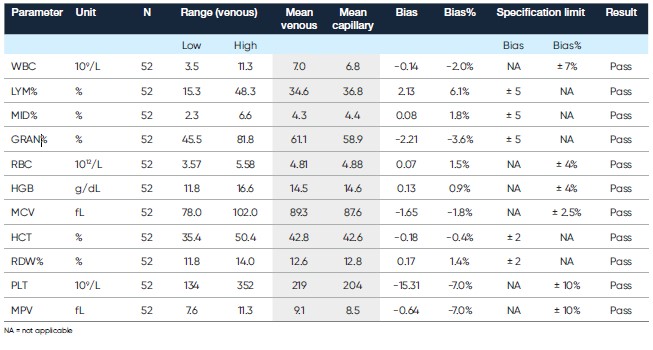
Table 3. Comparison of capillary blood collected in micro-pipettes and analyzed in MPA mode versus venous blood collected in bullet tube and analyzed in OT mode (7)
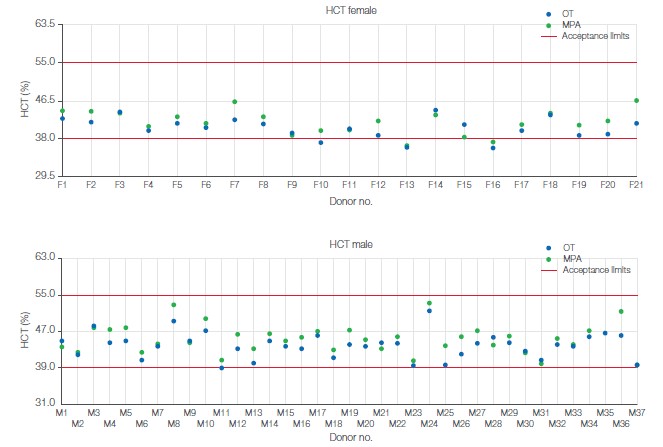
Fig 4. Sample analysis results for (A) male and (B) female donors. Requirement for donor acceptance at BloodWorks was an HCT value between 39% and 55% for male donors and between 38% and 55% for female donors.
Blood bank application for PLT concentrates
The Medonic M32 blood bank application resides in the Medonic M32 analyzer as a special analysis profile. The factory default blood bank profile is named PLT-C (i.e., PLT concentrate). This profile is pre-installed in the instrument and can be activated by the service technician upon delivery of the instrument (Fig 5). The PLT linearity range in the PLT-C profile is 10–5000 × 109/L, as compared with 10–1800 × 109/L in the normal blood profile. RBC concentrates are analyzed in the normal blood profile, with a linearity range of 0.30– 7.00 × 1012/L. Plots of recovered versus theoretical values show good conformity over the linearity range for both PLT and RBC concentrates (Fig 6). The results indicate that Medonic M32 can be a useful tool for efficient control of donated blood products.
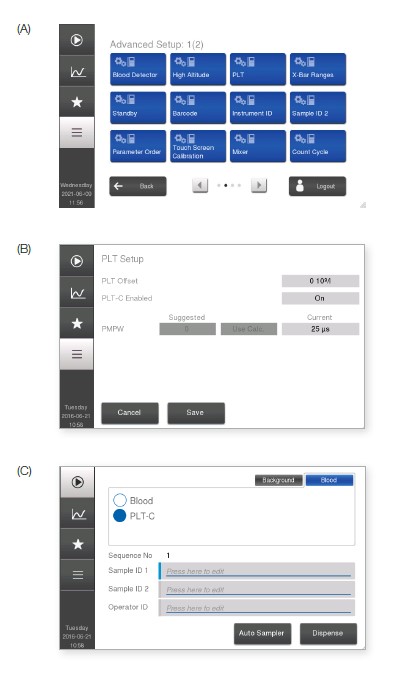
Fig 5. PLT-C profile is available in all analyzers with software version 1.3 or higher. (A) The profile is activated in Advanced Setup. (B) After entering the login code, go to PLT Setup menu and (C) enable PLT-C. In the Start menu, the PLT-C profile will thereafter be available as an option.
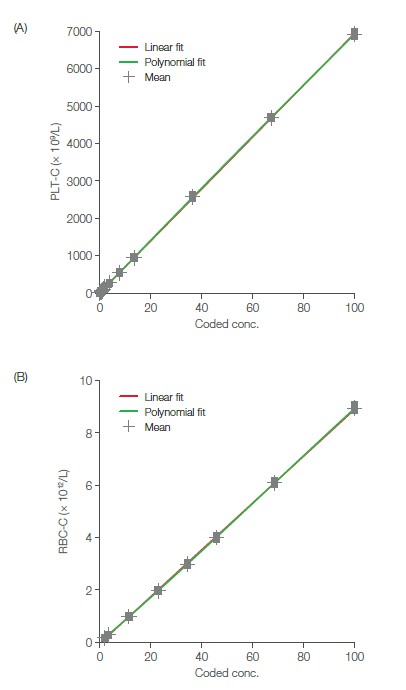
Fig 6. Recovered vs theoretical values for (A) PLT linearity and (B) RBC linearity (8). PLT concentrates were analyzed using the PLT-C profile, while RBC concentrates were analyzed using the normal blood profile.
Conclusion
Blood transfusion safety depends on many factors, including procedures for donor management, blood product manufacturing, and blood transfusion to recipients.
Hematology testing plays an important role at all these stages, from donor suitability assessment and quality control of the blood product to clinical assessment of the recipient.
Studies of the use of Medonic M32 automated analyzer in donor screening and evaluation of PLT concentrates show that this system can be a valuable tool for rapid and accurate
hematology testing in blood bank and transfusion center applications.
References
1. Guide to the preparation, use and quality assurance of blood components. European Committee on Blood Transfusion,
20th Ed (2020).
2. Heyes at al. A single unit transfusion policy reduces red cell transfusions in general medical in-patients. QJM 110, 735–739 (2017).
3. Hitzler, W.E. Single-Donor (Apheresis) Platelets and Pooled Whole-Blood-derived Platelets – Significance and Assessment
of both Blood Products. Clin Lab, 60, S1–S39 (2014).
4. Blood Donor Selection: Guidelines on Assessing Donor Suitability for Blood Donation. World Health Organization (2012).
5. Sarma, P.R. Red Cell Indices. Chapter 152 in Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths (1990).
6. Handbook: The Clinical Use of Blood. World Health Organization (2002).
7. Application note: Comparison of capillary and venous blood samples on Medonic M32 hematology analyzer. Boule Diagnostics, 31782, Edition 1 (2019).
8. Application note: Medonic™ M32 hematology analyzer helps ensure secure and efficient use of blood donations. Boule Diagnostics, 31881, Edition 1 (2019).
Disclaimer
The results and conclusions presented in this work are valid for this work only. Other conditions and assumptions could have significant impact on the outcome.
Medonic M32 from Boule Diagnostics is an automated hematology analyzer for in vitro diagnostic use under laboratory conditions. Boule products do not make diagnoses on patients. Boule intends its diagnostic products (systems, software, and hardware) to be used to collect data reflecting the patient’s hematological status. This data, in conjunction with other diagnostic information and the evaluation of the patient’s condition, can be used by a trained clinician to establish a patient’s diagnosis and to define clinical treatment.
Acknowledgement
We thank BloodWorks Northwest Donation Center, Seattle, WA, USA for kindly sharing study data.
PDF download
White paper: Whitepaper_Blood cell bank application_WP_36366-4