Hematology analyzers: 3-part or 5-part, that is the question
Automated hematology analyzers are frequently used in clinical laboratories to assess and monitor health condition of patients. Although 3-part hematology analyzers provide sufficient information for most clinical settings, trends show an increased interest in 5-part instruments.
While 5-part analyzers offer improved assessment of white blood cells, 3-part instruments offer great cost benefits. Here, we describe clinical settings, where 3-part differentials and 5-part differentials can provide different advantages.
Introduction
A complete blood count (CBC) is usually the first test requested by a physician to evaluate a patient’s general health status. CBC is used to measure the oxygen-carrying red blood cells (RBC), the platelets (PLT) that help clot the blood, and the white blood cells (WBC) of the immune system. As part of the CBC, a WBC differential is conducted. Traditionally, a laboratory technician uses a microscope to manually count 100 WBC and differentiate them into neutrophils (NEU), lymphocytes (LYM), monocytes (MONO), eosinophils (EOS), and basophils (BASO). Each cell type is reported as a percentage of total WBC, and a shift in the percentage indicates a condition.
In normal blood, about 60% of the cells are neutrophils. Neutrophils help fight bacteria (and fungi), and a high count (> 85%) indicates a bacterial infection. Lymphocytes, accounting for about 30% of all WBC, help fight viruses.
A high lymphocyte count can therefore be an indication of a viral infection. The last 10% comprises monocytes, eosinophils, and basophils. These cell types are typically associated with allergies or parasite infections. A high number of monocytes (2%–8% normal), for example, can indicate a chronic inflammatory disease or a bacterial infection, whereas high eosinophil counts (1%–4% normal) give an indication of asthma, an allergic reaction, or a parasite infection. A high number of basophils (0.5%–1% normal) is typically associated with inflammatory reactions, especially those causing allergic symptoms. High numbers of the WBCs can also be an indication of certain forms of cancers, such as leukemia or
lymphoma.
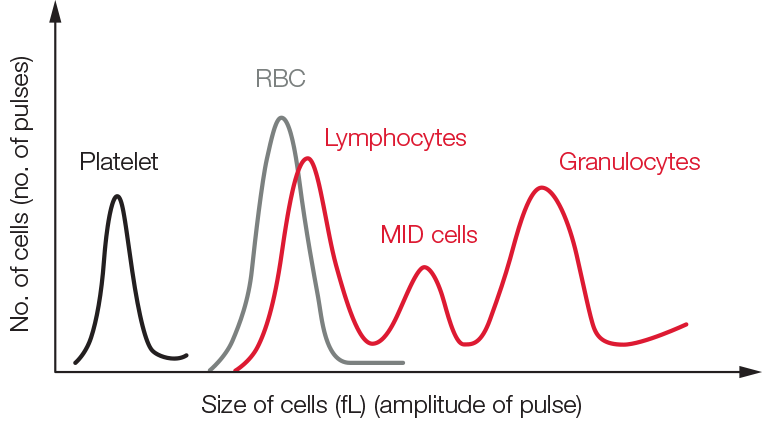
Fig 1. Test result from a 3-part hematology analyzer visualized in a histogram.
Automated hematology analyzers
In today’s general screenings, CBC tests are performed using automated hematology analyzers. In addition to reporting RBC, PLT, and WCB counts, an analyzer also measures the oxygen-containing hemoglobin (HGB) and determines a range of other parameters such as the mean cell volume (MCV), PLT width distribution, and hematocrit (HCT), that is, the red blood cell-to-plasma ratio. Hence, an automated analyzer can provide much more information than a manual count.
A 3-part instrument commonly uses impedance to differentiate WBC into granulocytes (mainly neutrophils, but also eosinophils and basophils), lymphocytes, and MID cells (mainly monocytes) based on cell size (Fig 1). Each cell passing through the aperture causes a drop in the electrical current (a pulse). The number of generated pulses correlates with the number of cells, whereas the size of the pulse is related to the cell size (Fig 2).
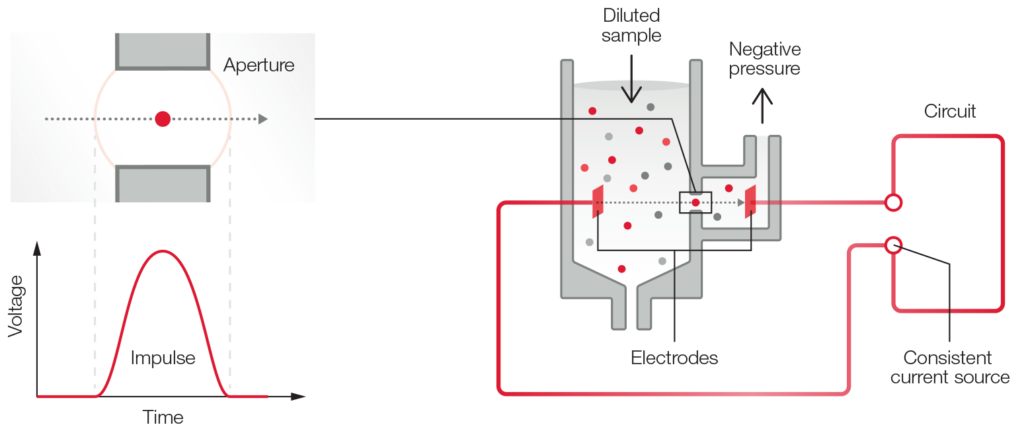
Fig 2. The principle for measuring changes in the electrical impedance produced by a cell passing through an aperture.
In addition to impedance, a 5-part instrument employs the principle of flow cytometry to differentiate WBC into their five major sub-populations—neutrophils, lymphocytes, monocytes, eosinophils, and basophils—based on cell size and complexity (granularity) (Fig 3). In flow cytometry, cells are forced to flow in a single file through the aperture by a sheath fluid, created by a fast-moving diluent that surrounds the slow-moving sample (Fig 4A). A laser beam is passed through the sample, and when a cell passes through the sensing zone, the light is scattered and measured by a photoconductor that converts the light into an electrical impulse. The number of generated impulses correlates with the number of cells, whereas the light scatter is used to determine cell granularity, shape, and size.
(Fig 4).
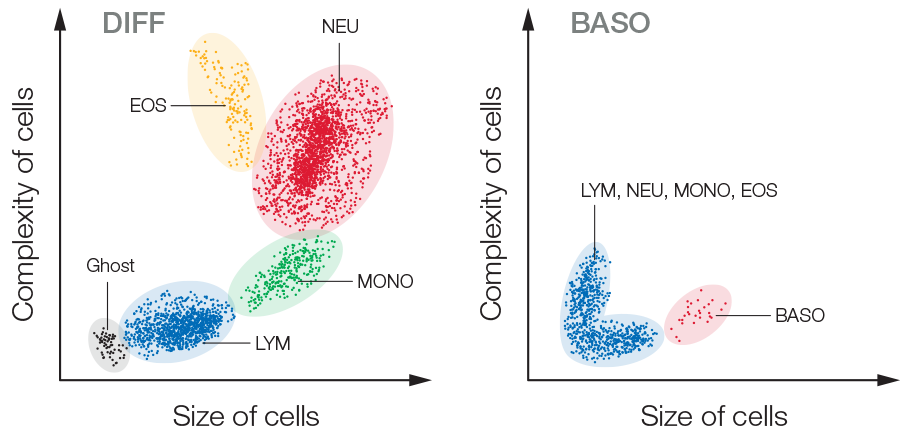
Fig 3. Test results from a 5-part hematology analyzer are visualized in a 4-part differential scattergram and a separate scattergram for BASO. Ghost = nucleated RBC, lyse-resistant RBC, and platelet clumps.
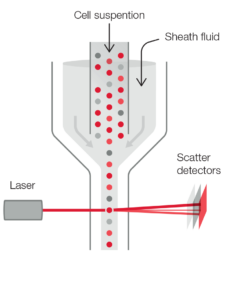
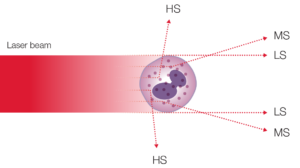
Fig 4. (A) Laser-based flow cytometry for 5-part differential of white blood cells. (B) Three-angle laser-scatter method, where the low angle signal (about 1° to 5°) represents the cell volume information, the middle angle signal (about 7° to 20°) represents the cell nucleus information, and the high angle signal (about 90°) represents the cell nucleus and cytoplasm information.
3-part versus 5-part analyzers
Although each WCB sub-type provides information that helps diagnose blood-related conditions, a 3-part instrument will provide sufficient information for the typical physician office laboratory (POL). With a simple CBC, the neutrophil and lymphocyte counts will answer the question of a viral infection or a bacterial infection that can be treated with antibiotics (Fig 5).
For specialty laboratories, however, a 5-part instrument can provide a more detailed and targeted assessment of the blood status. To distinguish eosinophils and basophils from neutrophils, for example, a 5-part differentiation is mandatory (Fig 6).
However, the cost for a typical 5-part instrument can be two to three times higher (20,000 to 50,000 USD) than for a 3-part instrument (less than 10,000 USD). In addition, a 5-part differential often requires more reagents than a 3-part differential, also increasing the cost per test from below 1 USD/test for a 3-part differential to 1.5 to 3 USD/test for a 5-part differential.
Yet, government reimbursements commonly do not consider whether WBCs are differentiated into 3- or 5-parts. Hence, 5-part instruments are typically used by oncology or allergy clinics that can justify the need for eosinophil and basophil counts.
Depending on local policies, samples that are flagged as abnormal commonly require microscopical examination to confirm the results obtained with the analyzer, regardless of instrument type. For this, blood smears are typically sent to a hematology laboratory for a manual count. According to many hematology laboratories experienced in blood smear examinations, the number of samples that requires manual examination can be greatly reduced with the more detailed information on the blood status provided by a 5-part differential. If about 30% of the total number of samples generates a suspicious flag with a 3-part instrument, and thus will require manual examination, the more detailed information provided by a 5-part instrument can reduce this number to about 20%, ultimately decreasing laboratory time and cost for shipping of samples (Fig 7).
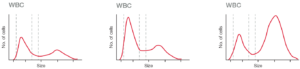
Fig 5. Histograms from (A) a normal sample, (B) a sample from patient with a virus infection, and (C) a sample from patient with a bacterial infection.

Fig 6. (A) Abnormal 3-part histogram, where lymphocytes cannot be differentiated from MID cells, and MID cells not from granulocytes. (B, C) Scattergrams from a 5-part instrument, showing a 4-part differential with a clearly separated eosinophil population, and the basophile population displayed in a separate diagram.
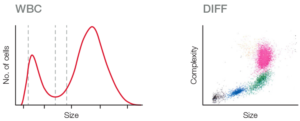
Fig 7. (A) A 3-part histogram, showing a lymphocyte peak that gives suspicion of finding smaller than normal lymphocytes, possibly caused by nucleated RBC that could not be distinguished from lymphocytes. (B) A 4-part differential that shows no flags and well separated cell populations, giving no suspicion of immature cells, resulting in an approved sample.
For general screenings, however, and when reimbursement is the same irrespective of 3-part and 5-part differential, a 3-part instrument can contribute to an improved laboratory economy by offering the caregiver a greater profit opportunity. In addition, a 3-part instrument based on robust impedance technology might require less maintenance than a 5-part instrument, which also includes a more sensitive laser-based measurement technology.
Conclusion
For most cases, the same decisions can be made with a 3-part hematology analyzer as with a 5-part analyzer. Typically, a 3-part instrument is also quicker and cheaper. If abnormal values are obtained, microscopy is still required, irrespective of instrument type. Although 3-part differentials provide excellent precision for general screenings, accuracy is improved with 5-part differentials for abnormal samples, reducing the number of manual blood smears.
Regardless of the choice of a 3-part or a 5-part instrument, of utmost importance is the ability of the analyzer to detect and flag for abnormal samples.
PDF download
White paper: Whitepaper 3p or 5p hematology analyzers
