Boule hematology solutions: total quality concept - from insights to solutions
Boule total quality concept spans all the way from initial insights of user needs to the support of our hematology solutions through their lifetime. With representatives and partners all over the world, we learn every day and share this knowledge throughout our organization. This approach allows us to provide solutions that holds a quality and robustness that enable decentralization of hematology testing to shorten time from blood collection to reporting of time-critical results.
Supporting health for everyone everywhere
A complete blood count (CBC) is on WHO’s essential diagnostics list (EDL) of test for which results are time-sensitive when used in emergency or critical care (1). Decentralized CBC testing can be especially critical in delivering urgent and appropriate health services in peripheral communities, disaster areas, and overcrowded areas (2). Supporting initial disease investigations as well as patient monitoring of disease progression and treatment efficacy, a CBC is one of the most performed laboratory investigations in medicine (3).
With the launch of one of the very first automated cell counters in the 50s, Boule’s legacy and expertise is in decentralized hematology testing (4). Today, our hematology solutions are available in more than 100 countries. Behind these solutions stands a solid, cross-functional, and hard-working team.

Meeting the specific needs of decentralized diagnostic testing
At Boule, we work closely with end-users to understand their needs. As can be seen from the product design, many features are tailored to fit the needs of the smaller laboratory. For example, our compact benchtop analyzers can be equipped with an autosampler that has a space-saving design to fit into confined areas. The autosampler is also designed to allow constant mixing of queued samples. For CBC testing, blood is collected in EDTA tubes that are gently mixed prior to analysis to prevent clotting. When working under the stressful conditions that can occur in emergency or critical care, it is easy to forget the mixing step. As decentralized hematology testing to large part is being used in fever investigations, a high erythrocyte sedimentation rate can be expected in many samples, and mixing is especially important for these samples.
The micro-pipette adapter (MPA) inlet is another feature of Boule hematology analyzers. The MPA function allows direct testing of capillary blood collected from a finger- or heel-prick in an EDTA-coated micro-pipette. This feature is especially useful for hard-to-puncture individuals and other patients from which a venous blood sample is not available, for example, in pediatrics, geriatrics, or oncology. The MPA inlet does not demand any phlebotomy expertise or equipment, or any sample preparation such as mixing, before analysis.
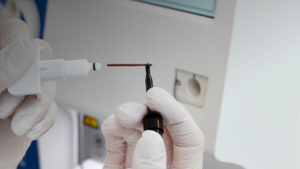
Boule hematology analyzers MPA function
Quality by design
Having in mind that a decentralized testing facility can be located at a far distance from service functions, Boule analyzers are also designed for robustness. As an example, the analyzer fluidic system is designed without moving parts, which can break or demand frequent maintenance, or syringes that can get clogged or cause leakages.
To provide measurement quality comparable to the centralized testing facility, Boule hematology analyzers are designed with a high-precision shear valve and reagent pipettes with optical sensors that enable precise sample aspiration and dilution. The measuring pipettes are equipped with liquid start and stop sensors to ensure that an absolute volume is used for counting.
An automated hematology system comprises both the analyzer as well as it dedicated consumables. At Boule Diagnostics, reagents are designed and developed in conjunction with the analyzer to provide optimized performance and enhanced serviceability of the complete system. Reagents and analyzer measurement technologies, including the analysis algorithms, are fine tuned to each other to produce the most accurate patient results. Formulations, dilutions, mixing, and reaction kinetics are all carefully matched and optimized to work together.
Field quality assessment
One of the most important elements of a complete hematology system is the quality control (QC) material. Like the reagents, the control and calibrator cell populations are matched to the analyzer measurement technologies and analysis algorithms. The QC functionality of the analyzer software allows plotting the results for controlled parameters over time in, so called, Levey-Jennings (L-J) diagrams to monitor agreement with assay values for the specific control lot. Results for the MCV, MCH, and MCHC parameters from routine testing can be plotted in, so called, X-bar diagrams to ensure no drift of these parameters over time.
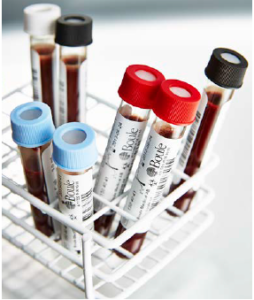
Boule´s Controls and Calibrators products are provided as part of the Boule Total Quality Concept to ensure performance of our hematology analyzers.
Consistent product quality that meets expectations
Boule uses manufacturing techniques that ensure the highest level of quality. Built on Swedish innovation and with many years of experience in manufacturing of hematology systems, our facility in Stockholm, Sweden is one of the most experienced in the business. Our team of engineers, technicians, and quality professionals are highly skilled in managing supply chains as well as assembly and testing of systems based on multiple complex technologies, including electronics, fluidics, and chemistry. To ensure quality, most of the manufacturing is done in-house, including all key assembly steps and testing. For the same reason, most key components are sourced from nearby and carefully chosen suppliers.
We adhere to strict regulatory compliance guidelines, allowing us to manufacture robust products that our customers can have the utmost confidence in. Coordinating consumables with analyzers allows us to provide a complete hematology system solution for customers around the globe. Our Quality Management System ensures full compliance with ISO 13485, EU IVDR, and US FDA QSR, offering high quality and reliability in the IVD market. By adhering to these stringent regulations, we deliver consistent product safety, performance, and effectiveness, significantly reducing the risk of non-compliance. Our clients consistently praise our regulatory affairs support, highlighting our proactive guidance, rapid response times, and deep expertise in navigating complex regulatory landscapes. Partnering with us means peace of mind, knowing that your products meet global standards while benefiting from our top-tier regulatory expertise.
When our products reach the clinic, our work has just begun
All customer orders are inspected, and nothing will be shipped that does not pass. Our Order & Logistics team assists worldwide customers with product information, order and shipping details, and organize workflows to meet customer time frames. We process incoming orders and manage transportation and physical logistics of all outgoing shipments to ensure goods arrive on time.
Boule reaches customers in over 100 countries through a distributor network of 200 locally based distributors that market, sell, and service our products. In addition to the product safety requirement, it is important for distributors to help build an infrastructure that gives patients safe and accurate diagnostics. Boule certified distribution partners act as key allies in delivering first-class solutions.
Although Boules hematology analyzers are designed to be robust and require minimal maintenance, the service organization is a key factor for an overall high-quality output. Therefore, Boule has a highly dedicated professional service team with service engineers positioned in every market as well as in the headquarter in Sweden. This team serves all the partners and end users around the world with technical inquiries, to ensure minimized downtime and maximized quality to leverage Boule’s vision to improve health for everyone, everywhere.
We will be present throughout the instruments’ lifetime to offer service, support, and training to end-users. Through Boule Academy, we are building a comprehensive online training, adding new digital tools to be better and faster, to meet the demands of today as well as tomorrow.
Learn more
We are customer-oriented in everything we do, to add value and to safeguard a quality that meets customer needs and expectations. We call it the Boule total quality concept. Your Boule team is there for you. Providing versatile, high-quality diagnostic solutions for everyone, everywhere.
Read more about the Boule total quality concept.
References
- WHO Technical Report Series, No. 1053: The selection and use of essential in vitro diagnostics: report of the fourth meeting of the WHO Strategic Advisory Group of Experts on In Vitro Diagnostics, 2022 (including the fourth WHO model list of essential in vitro diagnostics). Geneva: World Health Organization (2023).
- Elrobaa et al. The Role of Point-of-Care Testing to Improve Acute Care and Health Care Services. Cureus 16(3): e55315. DOI 10.7759/cureus.55315 (2024).
- El Brihi and Pathak. Normal and Abnormal Complete Blood Count With Differential. Bookshelf ID: NBK604207, StatPearls Publishing, Treasure Island, FL (https://www.ncbi.nlm.nih.gov/books/NBK604207/, accessed 2024-09-03).
- Celloscope automated cell counter (https://en.wikipedia.org/wiki/Celloscope_automated_cell_counter, accessed 2024-09-03)