Clinical utility of red blood cell indices in anemia investigations
This paper provides a general description of the utility of the red blood cell (RBC) indices of a complete blood count in anemia investigations. Anemia can be caused by a range of conditions, and symptoms are often non-specific. To help identify the underlying cause, anemias are classified according to the size of the red cells, as being normocytic (normal mean cell volume), macrocytic (increased mean cell volume), or microcytic (decreased mean cell volume).
Laboratory testing therefore begins with a complete blood count. Red blood cell indices such as the mean cell volume (MCV) and the distribution width (RDW) of the cells are especially useful, as these parameters often become abnormal before anemia becomes noticeable in other routine testing.
Introduction
Anemia is a serious health problem, especially affecting children; menstruating young girls and women; and pregnant and postpartum women. WHO estimates that 40% of children in the age of 6–59 months, 37% of pregnant women, and 30% of women in the age of 15–49 years worldwide are anemic. The cause of anemia can range from nutrient deficiency (e.g., iron or vitamin B12 deficiencies), an infectious disease (e.g., malaria, tuberculosis, or HIV), inflammation, chronic disease (renal or liver diseases), or an inherited red blood cell disorder (e.g., thalassemia and sickle cell disease) to loss of blood (e.g., due to ulcer or trauma). Anemia manifests in a range of non-specific symptoms such as fatigue (weakness, tiredness) or dyspnea (shortness of breath).
Anemia definition
Red blood cells use iron-containing hemoglobin (HGB) molecules to carry oxygen from the lungs to the body’s tissues where it is exchanged for carbon dioxide, which is transported back to the lungs to be expelled. Low HGB levels can be caused by a reduced number of RBCs due to an impaired production or premature destruction (hemolysis) or these cells. A reduced number of RBCs can also be caused by a loss of blood (acute or chronic hemorrhage). Lost blood is rapidly replaced by the body with water from tissues outside of the bloodstream to maintain the blood pressure. As consequence, the blood is diluted and the number or RBCs per volume of blood is reduced. A person is considered anemic if their blood HGB level is below what is considered normal for a healthy person. WHO defines anemia as HGB levels below 12 g/dL in women and below 13 g/dL in men. However, the normal reference range can vary with factors such as age, ethnicity, and physiological status (e.g., pregnancy) (1).
Common causes of anemia
Vitamin B12 and B9 (folic acid) are important cofactors for normal RBC proliferation and maturation. A deficiency in any of these factors can result in a defect DNA synthesis, resulting in large oval-shaped RBCs (macro-ovalocytes). Vitamin B12 and B9 deficiencies can be due to insufficient intake (e.g., malnutrition), increased consumption (e.g., during pregnancy), or impaired uptake (e.g., inherited, or due to gastric bypass or certain medications). Evaluation of serum B12 and RBC folate levels can be used to distinguish vitamin B12 and B9 deficiencies from other macrocytic anemias (2).
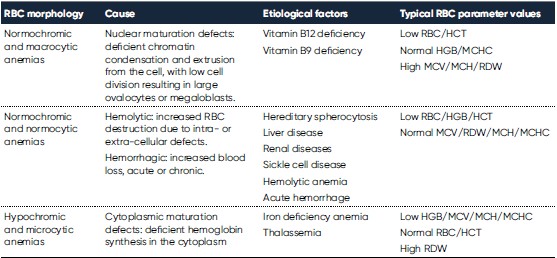
Hemoglobin consists of four subunits, each comprising one globulin polypeptide that helps keeping the HGB protein soluble and one heme group to which an iron atom is attached. Iron is an important component for the oxygen-carrying capacity of hemoglobin. A deficiency can occur in case of blood loss (e.g., due to an ulcer, menstruation, or blood donation), insufficient iron intake (e.g., malnutrition), increased consumption (e.g., during pregnancy), or impaired iron uptake (e.g., in celiac disease). In case of defective hemoglobin synthesis due to iron deficiency, the cells are unevenly small (microcytic), and anisocytosis (increased RDW) can be the first laboratory finding that indicates anemia (Fig 1).
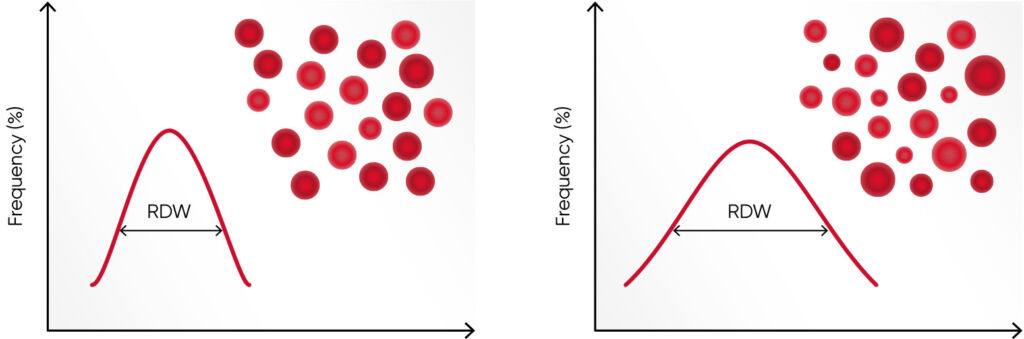
Values of the RBC parameters are often similar between iron deficiency anemia and thalassemia. However, as the RBC count in thalassemia can be normal or slightly elevated, compared with an iron deficiency, where RBC count can be slightly below normal, the Menzer index (MCV [fL] /RBC count [× 1012/L]) can be useful to differentiate iron deficiency anemia (MI > 13) from thalassemia (MI < 13) (3). Additional tests, such as serum iron, ferritin, and transferrin levels and total iron binding capacity (TIBC) are typically determined in parallel with the CBC test to distinguish the two (4).
Hemolytic anemia is defined as the destruction of the RBCs before their normal lifespan (approx. 120 days). The causes can be many, for example, drug-induced, antibody-mediated, or due to trapping and phagocytosis or fragmentation of cells with abnormal morphology, which can be inherited (e.g., thalassemia and sickle cell disease) or induced by an infection (e.g., malaria). An increased RBC production rate is the bone marrow’s response to anemia and is commonly detected as an increased reticulocyte percentage of the total number of RBCs. Consequently, a reticulocyte count is often requested in case of evidence of hemolysis on the blood smear, such as an increase in RBCs of a bluish color (polychromasia) due to residual RNA (5).
In acute hemorrhage, on the other hand, the RBC level decline, whereas the bone marrow’s output of reticulocytes (that later lose their RNA and mature into RBCs) remains stable. The reticulocyte percentage of the total number of RBCs can therefore be falsely elevated in early post-hemorrhage phase. However, modern automated hematology analyzers can report reticulocyte count in absolute numbers with high accuracy as complement to the relative reticulocyte count as the percentage of the total RBC count.
Utility of CBC testing in anemia investigations
A complete blood count (CBC) is the main test for identification of anemias. The RBC components of the CBC are:
• Red blood cell count (RBC)
• Hemoglobin (HGB)
• Hematocrit (HCT)
• Mean cell volume (MCV)
• Red blood cell distribution width (RDW)
• Mean cell hemoglobin (MCH)
• Mean cell hemoglobin concentration (MCHC)
Whereas RBC (and HCT) and HGB are used for diagnosis of anemia, the RBC indices MCV, RDW, MCH, MCHC are valuable in the morphological classification of anemia (Table 1). While MCV and RDW are used to identify the type of anemia, the MCH and MCHC can be used to confirm the findings.
As an elevated HGB value is expected to correlate with an elevated RBC count, and an increase in the number of RBCs is likely to correlated with an increase in the HCT value, another useful tool to ensure that the interpretation of the RBC parameters is correct is the “rule of three”:
RBC (× 1012/L) × 3 = HGB (g/dL)
HGB (g/dL) × 3 = HCT (%)
The following steps can be used for interpretation of the RBC parameters of the CBC (5):
1. Assess HGB value relative to reference interval to determine severity of anemia.
2. Assess MCV relative to reference interval to determine class of anemia (macrocytic, normocytic, or
microcytic).
3. Assess the MCHC relative to the reference interval (hypochromic or normochromic) – an increase can
point to spherocytes (spherical RBCs without the central concavity).
4. Interpret the RDW relative to the reference interval (low/moderate or pronounced anisocytosis).
5. Examine the RBC morphology under the microscope and correlate findings with instrument
parameters. At the same time, review for additional findings that may support diagnosis.
6. Examine RBC, HCT, MCH, and calculate the “rule of three” to ensure that the interpretations are
correct.
7. Use related test results when available to verify CBC findings and for additional diagnostic
information.
8. Correlate RBC parameters with WBC and PLT results for diagnostic significance.
Among factors that can affect the results from the automated cell count are lipemia or elevated WBC count (falsely elevated HGB value), blood clotting (falsely low HCT), and hemolysis (falsely elevated MCHC due to a disproportional drop in HCT versus HGB present in the plasma). The “rule of three” can be helpful to doublecheck the results if the MCHC value is within the reference interval.
Case studies
Macrocytic anemia
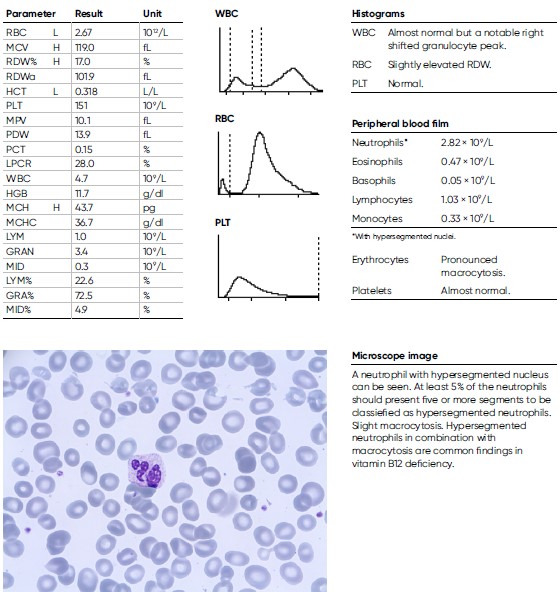
An 81-year-old man was noted for a moderate anemia, with a HGB level of 11.9 g/dL and a pronounced elevated MCV of 119 fL. He was considered for further investigation and other laboratory findings were CRP = 1.7 mg/L, folic acid = 5 nmol/L, Vitamin B12 = < 62 nmol/L, homocysteine = 41.7 μmol/L. The man was diagnosed with Pernicious anemia, a condition that manifests as low RBC due to that the intestines cannot properly absorb vitamin B12.
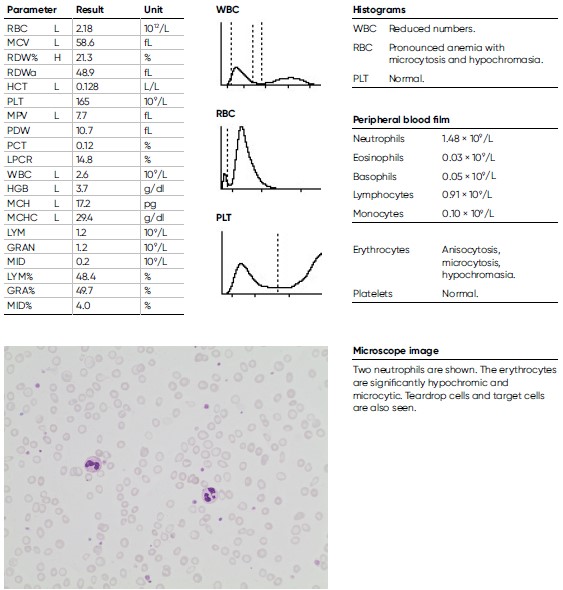
Microcytic anemia
A 16-year-old male teenager, previously healthy and engaged in sports besides going to school, experienced headache and deteriorating physical capacity during the last four weeks before visiting a general practitioner who found extreme anemia, with a HGB level of 3.7 g/dL and a low MCV of 58.6 fL. He also reported occasional blood in the stools. A very low ferritin level of 1 μg/L (reference interval 30– 400 μg/L) was later documented, indicating an iron deficiency anemia.
Sickle cell anemia
A 29-year-old man of African descent with sickle cell anemia (homozygote HbSS) experiences recurrent vaso-occlusive symptoms with pain in the extremities, due to obstructed microcirculation from RBCs, causing ischemic injury. He had been treated in hospital with hydration and opioid analgesics. The patient also had an episode of acute chest syndrome and pneumonia with a nadir HGB of 8.3 of g/dL and maximum CRP of 218 mg/L. Otherwise mostly stable HGB levels between 9.0–10.5 g/dL, with slight reticulocytosis and elevated lactate dehydrogenase (LDH) and bilirubin. It was decided to start treatment with hydroxyurea orally against the frequent pain episodes. A CT scan of his left femur (thigh bone) shows signs of a distal infarction.
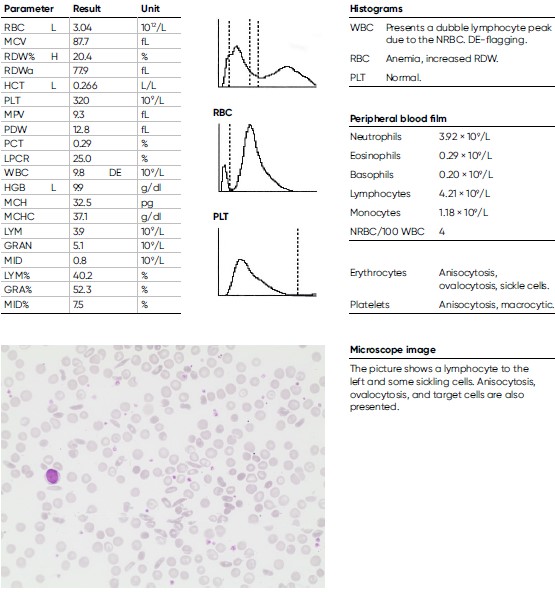
Infection-induced anemia
A 9-year-old male child arrived from the Immigration Health Care to the hospital with a history of coughing. He arrived alone to Sweden as a refugee from Eritrea and has been travelling for seven months and was tired and nauseous. He presents with an anemia, with an HGB level of 9.9 g/dL and CRP of 43 mg/L. The blood film shows Plasmodium vivax < 0.5%. He was treated with chloroquine and primaquine.
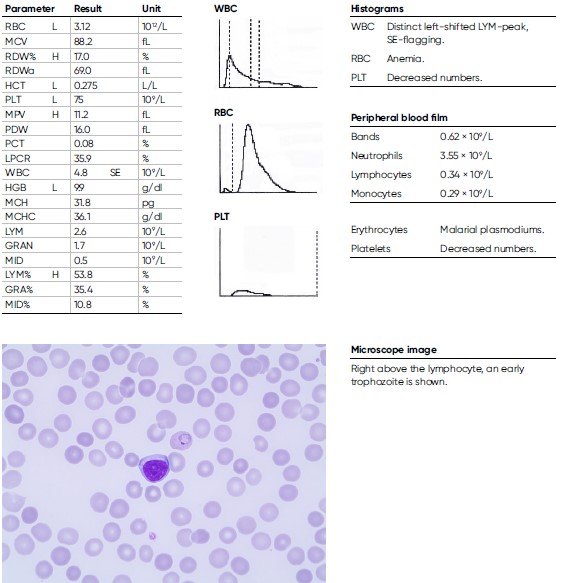
Conclusions
Anemia can have several causes and the symptoms are ambiguous. Yet, time for a correct diagnosis is often urgent, as a low hemoglobin level can result in unconsciousness, coma, or even death. Red blood cell indices are useful in classification of the anemia. A complete cell count is therefore the primary test requested in early anemia investigations.
References
1. WHO: Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011 (WHO/NMH/NHD/ MNM/11.1) (http://www.who.int/vmnis/indicators/haemoglobin.pdf, accessed 2023-05-05).
2. Moore and Adil. Macrocytic Anemia. StatPearls Publishing LLC, Treasure Island, FL, USA, Jan 2023.
3. Mentzer WC Jr. Differentiation of iron deficiency from thalassaemia trait. Lancet 1, 882 (1973).
4. Jameel et al. Differentiation of beta thalassemia trait from iron deficiency anemia by hematological indices. Pak J Med Sci 33, 665–669 (2017).
5. Doig and Zhang. A Methodical Approach to Interpreting the Red Blood Cell Parameters of the Complete Blood Count. Clin Lab Sci 30, 173–185 (2017).
6. Lippi and Plebani. Recent developments and innovations in red blood cells diagnostics. J Lab Precis Med doi: 10.21037/jlpm.2018.07.09 (2018).
7. Sarma, P.R. Red Cell Indices in Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition (Ed. Walker H.K., Hall W.D., Hurst J.W.), Boston: Butterworths (1990).
Disclaimer
The results and conclusions presented in this work are valid for this work only. Other conditions and assumptions could have significant impact on the outcome. Automated hematology analyzers from Boule Diagnostics are intended for in vitro diagnostic use under laboratory conditions. Boule products do not make diagnoses on patients. Boule intends its diagnostic products (systems, software, and hardware) to be used to collect data reflecting the patient’s
hematological status. This data, in conjunction with other diagnostic information and the evaluation of the patient’s condition, can be used by a trained clinician to establish a patient’s diagnosis and to define clinical treatment.
Acknowledgement
We thank Kristina Nilsson, Reg. Biomedical Scientist, and Sigward Söderström, Reg. Biomedical Scientist, for collecting, editing, and photographing material for the case studies. All material for these investigations had been taken from daily routine blood samples analyzed in the Hematology Laboratory, at the Department of Clinical Chemistry, Örebro University Hospital, Sweden. We also thank Maria Åström, Hematologist, MD, for clinical evaluation and Maria Sjöberg, nurse at the infection clinic, for assisting with patient data for the Section Parasitic infections. Cases are reviewed and diagnoses are adapted to the current WHO-classification by Helene Johansson, Reg. Biomedical Scientist, Sahlgrenska University Hospital, Göteborg.
