Quality assurance (QA) in the clinical laboratory comprises activities and procedures to ensure that the reported results are as correct as possible. While quality control (QC) as part of QA describes the process that ensures performance of the laboratory test, from sample collection to reporting of result, the QA program also includes external quality assessment (proficiency testing) and standardization.
Pre-analytical phase in the quality assurance
Connectivity simplifies interaction between the central laboratory and its satellite testing facilities. Bidirectional communication between the testing device and a central data management system, such as a laboratory information system, removes redundant data entry steps, and patient test results and quality control data can be automatically stored in the data repository together with metadata such as consumable lot numbers. Electronic data transfer also eliminates transcription errors related to manual entry.
The instructions for use of the hematology system describes which specimen receptacles to use for blood collection. The blood collection procedure recommended by the manufacturer is typically based on a standard procedure for collecting venous or capillary blood.
Pre-analytical errors constitute a large part of the total number of errors that may affect the cell count test results. It is therefore of utmost importance to follow manufacturer’s instructions and use standardized procedures for sample collection and handling prior to analysis. Spurious results can also be caused by the patient’s health status or treatment regimen.
Analytical phase in the Quality assurance
Internal quality control
In automated hematology testing, QC is done with The results for the control parameters are plotted over time in, so called, Levey-Jennings (L-J) diagrams to ensure agreement with assay values for the specific control lot. Results for the MCV, MCH, and MCHC parameters from routine testing is also plotted in, so called, X-bar diagrams over time to ensure no drift of these parameters over time. QC is monitored on a daily basis.
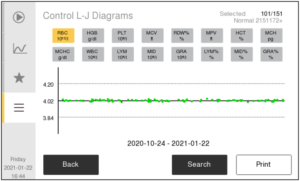
Levey-Jennings (L-J) diagrams
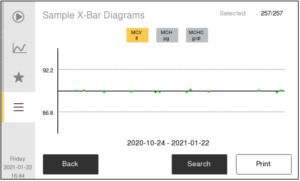
X-bar diagrams
Calibration
In case results from control materials indicate an unusual trend or shift of the analyzer performance, a calibration is conducted using assigned parameter values traceable to a reference method. A new calibration factor to obtain accurate results is calculated for each parameter by the analyzer. While a control is used to address random error and indicate whether the analyzer works precisely, the calibrator is used to measure and adjust systematic error to ensure the analyzer is working accurately.
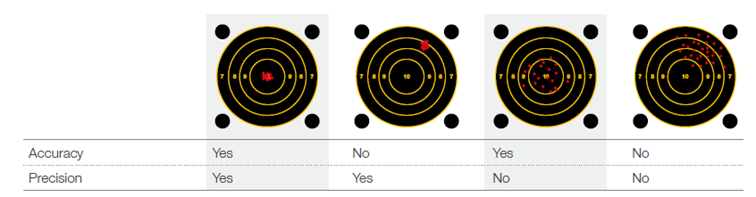
External quality assessment
To ensure inter-laboratory copmarability, laboratories can participate in
Post-analytical phase
The post analytical phase comprises steps such as data transfer; review and verification of results; and communication and interpretation of the results. After the pre-analytical phase, the post-analytical phase constitutes the second most affected by errors. As for the pre-analytical phase, electronic transfer of data to a laboratory information system can help eliminate transcription errors.
Automated hematology analyzers are analytically and technically complex, and the reported results comprise a range of parameters. In addition to the parameter values, the histograms and scatterplots constitute an important part of the results and should be carefully studied, as they provide valuable and important insights about the sample processed by the analyzer.
Laboratory accreditation
Laboratory accreditation by an accreditation body is a formal recognition earned by the testing laboratory, proving they are qualified, competent, and comply with international standards. Laboratory accreditation is mandatory by law for laboratories operating in certain countries, but voluntary in others. The aim of laboratory accreditation is to facilitate accurate and rapid diagnostics by reducing errors in the laboratory process, ultimately to improve efficiency of treatment.
Learn more about Quality assurance in hematology analysis
Learn more from the article